USask researchers probe drug’s potential ability to block pre-term labour
How can we decrease the occurrence of pre-term births in women? Can a cancer research drug help us find a solution? These are questions facing reproductive scientist Dr. Daniel MacPhee (PhD) and his research team at the University of Saskatchewan (USask).
By Arej Kondi
However broad they may seem, these simple questions come with very complex answers. For over 20 years, MacPhee and his colleagues have been working to understand the complexities and intricacies of reproduction and birth as they focus on pre-term labour and its causes.
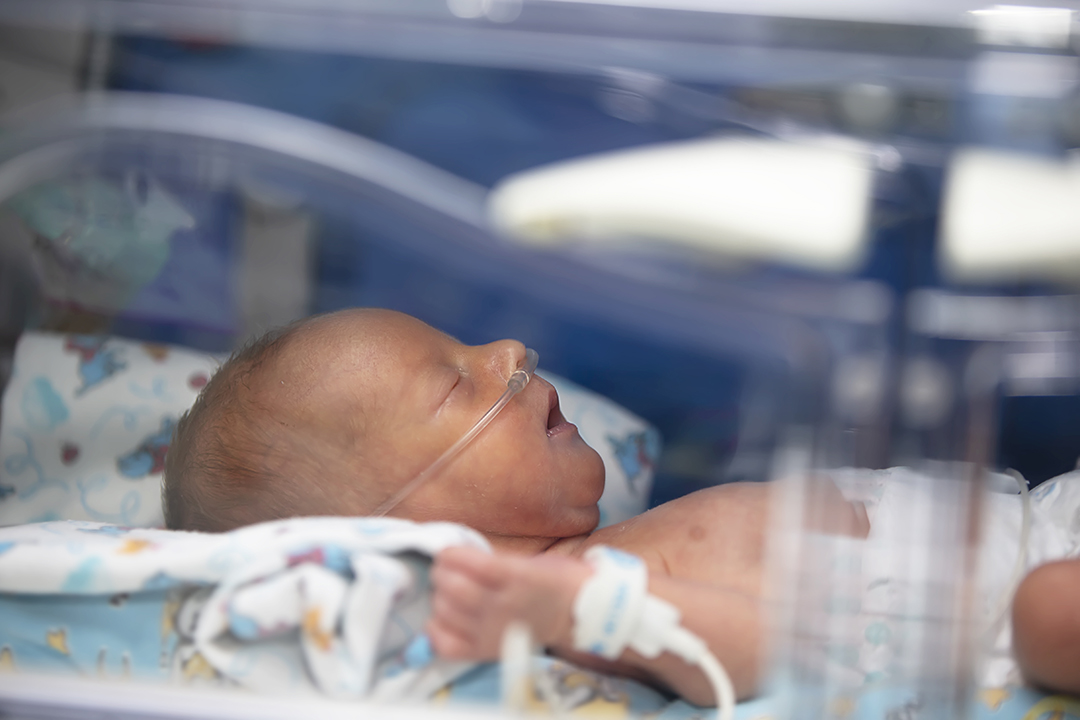
Pre-term labour annually affects about 15 million babies and is one of the leading causes of infant death. About 45 to 50 per cent of premature births happen spontaneously with no real known cause. In many cases, survivors of early birth (born before 37 weeks of gestation) end up living with one or more disabilities such as hearing loss, blindness, chronic lung disease or cerebral palsy.
Research in MacPhee’s lab has been especially focused on the myometrium — the middle layer of the uterine wall that transforms from a dormant muscle during pregnancy to the contractile (capable of shrinking or contracting) muscle that’s required for labour and delivery.
“Pregnancy is a stressful period of time,” says MacPhee, a professor of reproductive sciences at the Western College of Veterinary Medicine (WCVM). “The uterus undergoes a lot of changes and a lot of stress as a result of a growing fetus. We are particularly interested in mechanical force-like stretches. We are interested in how stress plays a role in regulating pregnancy, but specifically and more importantly the labour process itself.”
The researchers are diving deep into the cell as they investigate specific proteins that are involved in the labour process. Although many types of proteins play a role in childbirth, the small heat shock proteins are a stress protein family that’s of particular interest.
Labour requires an inflammatory activation of the myometrium which in turn starts phosphorylation — a process during which phosphate molecules are transferred to proteins. When this process activates a particular protein, it can trigger the signalling pathways associated with labour.
One particular protein, heat shock protein B1 (HSPB1), is of interest to the WCVM researchers since it’s highly expressed in myometrial cells toward the end of pregnancy and labour. They’ve determined that HSPB1 is highly phosphorylated and inflammation leads to its activation.
MacPhee is investigating the novel drug KRIBB3 to see if it can actually prevent the phosphorylation of HSPB1 and inactivate it with the goal of stopping the onset of inflammatory processes in myometrial cells.
“About 15 years ago, KRIBB3 was developed in South Korea. It is an organic compound and seems to be able to bind to the specific small heat shock protein we are interested in, and by binding to it, prevents the phosphorylation of that protein on specific residues,” MacPhee says.
“What was intriguing to me was how could we potentially use that drug to block phosphorylation of that protein and as a result look at what the downstream effects of that would be in our uterine muscle cells. That was the rationale for studying this drug.”
Researchers have also established that KRIBB3 disrupts the function of microtubules, substances that form part of the cell cytoskeleton and are involved in various cellular processes. KRIBB3 was originally investigated as a potential means of stopping cancer cell movement by deactivating stress proteins in cancer cells.
WCVM researchers are now investigating KRIBB3 to determine its toxicity as well as its ability to block the phosphorylation of the heat shock protein without damaging myometrial cells.
MacPhee hopes the drug may provide a new tool that researchers can use to manipulate the small heat shock protein's function. If so, it’s possible that this inhibitor, a drug that disrupts the cellular skeleton, could be a potential treatment for blocking pre-term birth — particularly if this specific heat shock protein is important for driving uterine contractions.
Perhaps the biggest challenge for MacPhee and his team is understanding how the results of this study and all of their other studies fit together.
“Multitudes of integrated pathways are involved in the labour process so until we know much more about these integrated pathways, we really can’t say we can block pre-term birth with a drug,” says MacPhee.
Once researchers can understand exactly how labour is regulated, they can work to design a strategy for blocking pre-term labour. After years of operating in discovery mode, MacPhee and his research team are continuing their work toward a solution that will save the lives of many newborns.
“Discover, find information, find pathways and proteins involved in the labour process, and maybe down the road we can target a particular pathway that will block pre-term birth,” says MacPhee.
The Natural Sciences and Engineering Research Council of Canada (NSERC) is providing financial support for this research study.
Arej Kondi, a fourth-year physiology and pharmacology honours student in the University of Saskatchewan’s College of Arts and Science, was a summer research student at the Western College of Veterinary Medicine (WCVM) in 2022. Her story is part of a series of articles written by summer research students at the WCVM.
