USask researchers identify promising protein candidate for metabolic disease treatment
A University of Saskatchewan (USask) research team’s discovery of the additional health benefits of an appetite-suppressing protein has doubled the potential for scientists to find new avenues for treating obesity and metabolic disorders in animals and people.
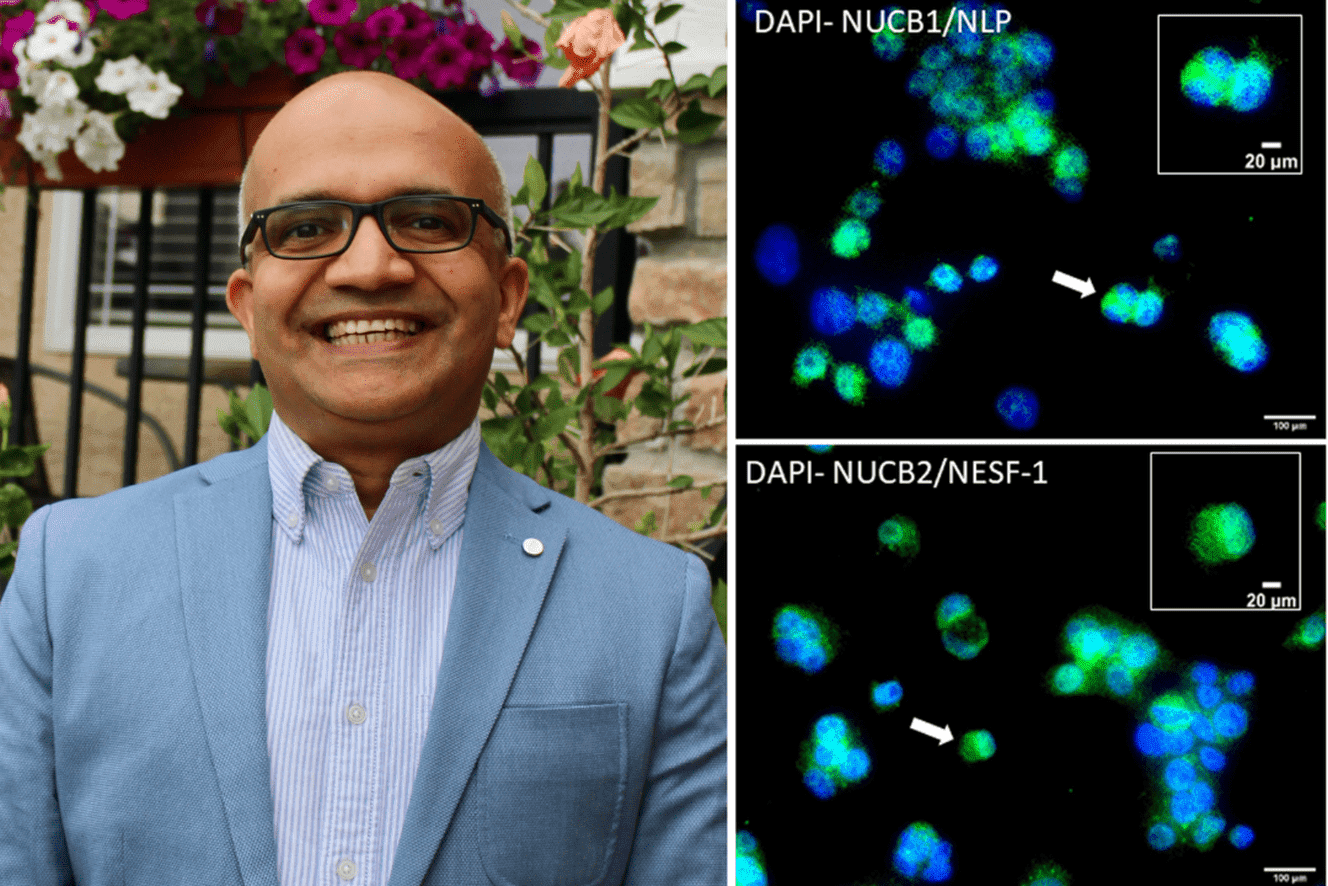
By Rigel SmithThe researchers’ findings, which were recently published in Nature Communications Biology, highlight their discovery of the lipid-lowering effects of nesfatin-1-like peptide (NLP). This newly identified peptide—or small protein—is a close relative of nesfatin-1 (NESF-1), which regulates feed intake and body weight.
“We found that both NESF-1 and NLP lower lipid (fat) accumulation in human liver cells,” said research team member Dr. Suraj Unniappan (PhD), the university’s Centennial Enhancement Chair in Comparative Endocrinology and a professor at the Western College of Veterinary Medicine (WCVM). The collaborative study involved researchers at the WCVM and USask College of Medicine.
While the lipid-lowering effect of nesfatin-1 was previously reported, Unniappan said identifying NLP and understanding its lipid-lowering capabilities in human cells represent new advancements in the field of endocrinology.
“We are far away from bringing these findings to bedside,” said Unniappan. “But we now have additional multiple targets available that could be explored for lipid disease treatment and therapeutic advancements.”
The research team’s discovery is hopeful news since there’s a lack of new therapies for many metabolic diseases—including non-alcoholic fatty liver disease (recently renamed as metabolic dysfunction-associated steatotic liver disease or MAFLD), which affects about 20 per cent of Canadians. A hormone-based drug was approved in the United States in March 2024, but so far, there are no drugs currently available in Canada exclusively for treating this disease.
Typical treatment plans for both humans and animals suffering from metabolic disease generally consist of diet and exercise changes to gradually lower body weight and reduce fat accumulation.
Unniappan and his research team have been at the forefront of nesfatin-1 research. Discovered in 2006 by a group of researchers in Japan, nesfatin-1 was initially recognized for its ability to suppress food intake.
The USask team went a step further than previous studies and successfully verified that genetic disruption of NLP leads to changes in genes involved in lipid metabolism in mice.
“We found that if you disrupt the gene that is the source of that protein [NLP] naturally present in these animals, then that actually leads to changes in lipid metabolism-associated genes,” said Unniappan.
Discovering such results—that administering NLP reduces lipid levels, while disrupting its production alters lipid metabolism—reinforces its pivotal role in metabolic regulation.
Unniappan and Dr. Atefeh Nasri (PhD), who completed her doctoral program at USask in 2023 and is now a post-doctoral fellow at Dalhousie University, collaborated with Dr. Scott Widenmaier (PhD), an assistant professor of anatomy, physiology and pharmacology, and an expert in metabolic disease at the USask College of Medicine. The team’s fourth member was undergraduate student Mateh Kowaluk.
Unniappan hopes that this new research can pave the way for further exploration of treatment options. He plans to work with collaborators to extend this research to more complex animal models—including rodents—and eventually studying larger animals such as cats and dogs. Like humans, these species also suffer from obesity and related metabolic disorders.
“It’s beautiful to know the same peptide can achieve so many meritorious health effects, that in combination have the potential to help both human and animal patients,” said Unniappan.
This research was supported by the Canadian Institutes of Health Research (CIHR) and the USask Centennial Enhancement Chair in Comparative Endocrinology.
Together, we will undertake the research the world needs. We invite you to join by supporting critical research at USask.
