
Tissue explants offer promising alternative for swine influenza research
Researchers at the University of Saskatchewan (USask) are exploring an innovative approach to swine influenza research that uses tissue explants as an alternative to conducting live animal trials.
By Allison KuzubTissue explants involve growing living tissues outside the body in a controlled culture medium. Dr. Susan Detmer (DVM, PhD), an associate professor at the Western College of Veterinary Medicine (WCVM), and her graduate student, Dr. Carlos Azeredo (DVM, PhD), are at the forefront of this research. They are validating new uses for the explant model that aim to align with the three Rs of research: reduction, refinement and replacement of live animal use.
Azeredo describes these explants as small sections of pig trachea that retain cellular structure and local immune components, more closely resembling the natural environment that the virus would encounter in a live pig.
Traditionally, influenza research in pigs has relied on the use of live pigs and cell culture models. While both methods provide valuable insights, the use of live animals comes with significant ethical and logistical challenges.
“Research using live pigs can be expensive, ethically concerning and time-consuming. Using explants significantly reduces the number of animals required for research, addressing ethical concerns and offering cost-effective benefits,” says Azeredo.
“Tissues can be harvested from healthy animals from other research studies or abattoirs if the timing and tissue handling can be co-ordinated,” adds Detmer.
Unlike cell cultures, trachea explants preserve a three-dimensional structure that more closely resembles natural organs. These explants can produce mucus and mount local immune responses, providing a more physiologically relevant model for studying influenza disease progression compared to immortalized cell lines that can’t replicate the complexity of bodily tissue functions.
Influenza A viruses (FluA) cause significant challenges in pig production, affecting animal health and welfare, food production costs and potentially public health. FluA viruses are well known for their ability to slowly and constantly acquire genetic mutations then rapidly changing by swapping genes with other FluA viruses. This scenario creates an ongoing need for scientists to develop and update vaccines and other control measures for FluA.
The global genetic variation of FluA in pigs and the similarity of some strains found in pigs and people creates an opportunity for new virus strains to be introduced in naïve populations — with the potential for the virus to be transmitted to humans and widely spread among global populations. In 2009, the H1N1 pandemic caused economic disruptions to the swine industry as well as widespread illness in humans and pigs. These viruses — described as 2009pandemicH1N1-like viruses — continue to circulate in both human and pig populations today.
A recent pilot study conducted by Detmer’s research team successfully demonstrated infection progression observed in live pigs using trachea explants infected with two Canadian viruses of 2009pandemicH1N1 origin.
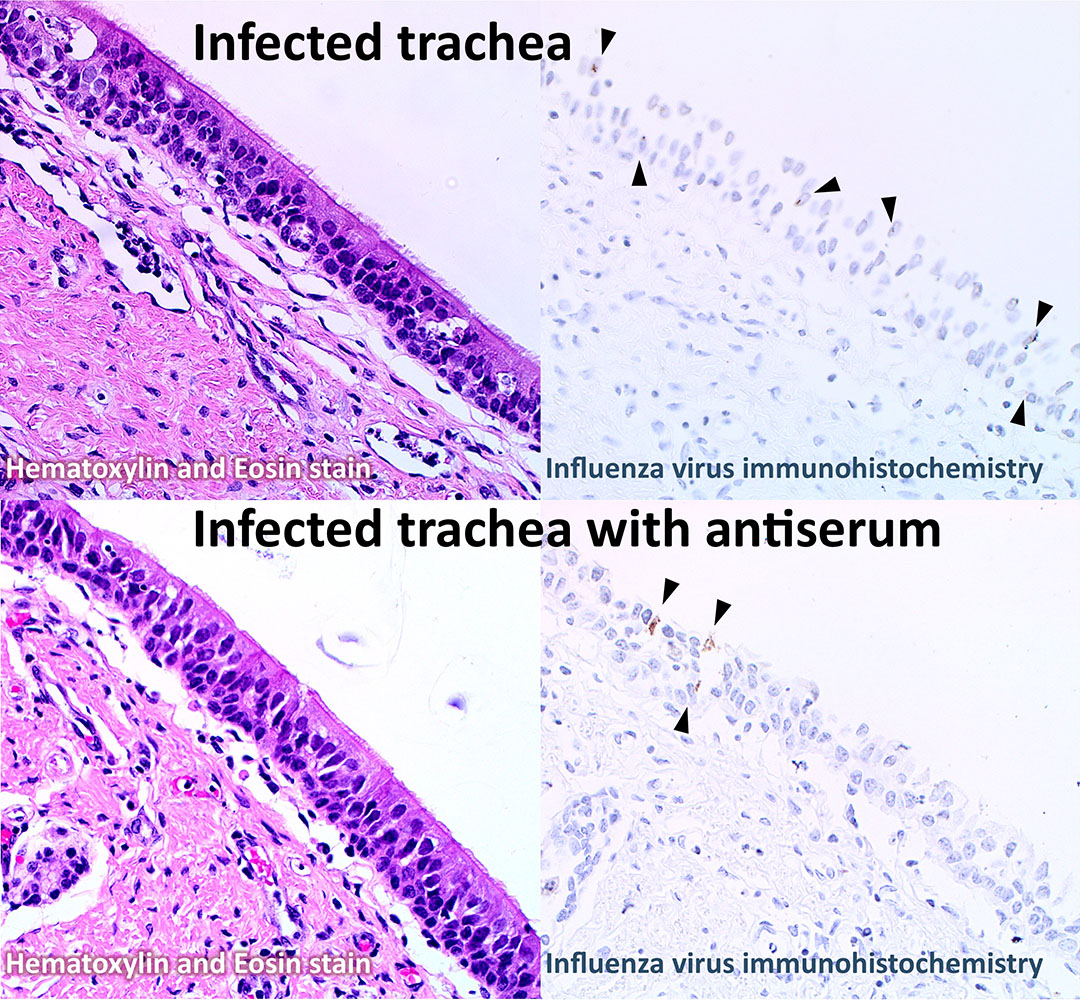
Researchers evaluated serum neutralization, a technique to assess antibody protection against virus infections, by mixing these viruses with serum from a pig vaccinated with the original pandemic strain, A/California/07/2009, which is available in a commercial pig vaccine. Infection with the neutralized virus was then compared to the virus alone.
This achievement marks significant supportive evidence of tissue explants being a reliable and effective research model.
“By examining the effects of different viruses on the tissue explants, the replication rate of the virus in the model, and testing the ability of serum antibodies produced by vaccination in pigs, and by comparing these results to traditional in-vitro assays, a more comprehensive assessment of a new vaccine or virus can be made,” says Detmer.
Although promising, tissue explants are not without limitations. Azeredo says it’s challenging to maintain tissue viability over extended periods, which is crucial for studying longer-term viral effects. Explants also don’t fully capture systemic interactions and whole-body responses as seen in live animals.
Despite these challenges, Detmer envisions explants as bridging the gap between in-vitro cell culture and live animal models.
“While I don’t see them as totally replacing animal experiments, I see us doing more in the laboratory, using fewer animals for research and at a lower overall cost while refining our experiments and research questions,” says Detmer.
WCVM researchers remain optimistic about the potential use of tissue explants as both an accurate and more ethically sound model for vaccine development and other infectious disease research. Their work is advancing the field of swine influenza research — plus exploring an alternative to other research methodologies that could help to reduce research expenses and improve animal welfare.
This research project was funded by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC-Discovery).
Allison Kuzub of Saskatoon, Sask., is a third-year veterinary student who worked as a summer research student in 2024. Her story is part of a series of articles written by WCVM summer research students.
Together, we will undertake the research the world needs. We invite you to join by supporting critical research at USask.
